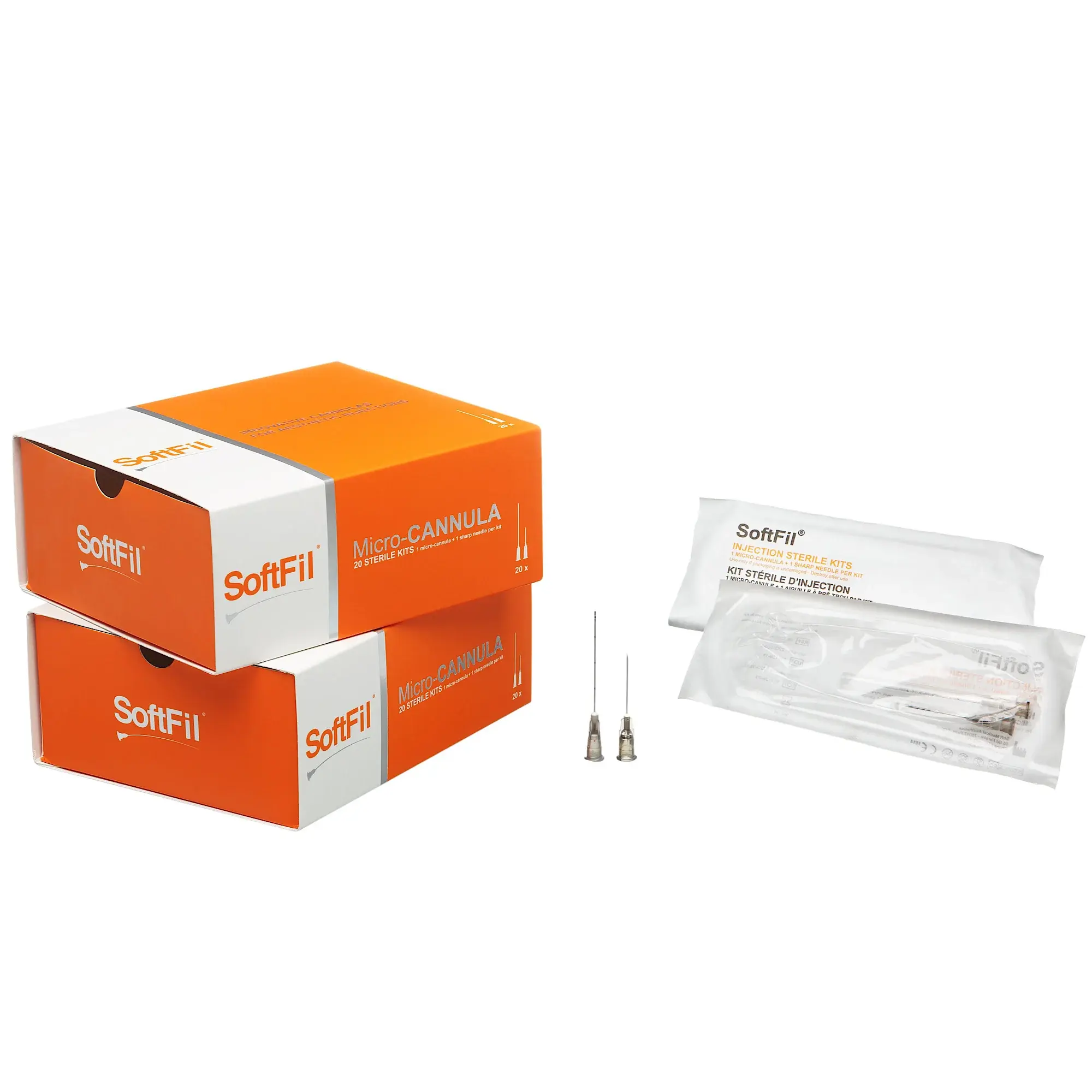
SoftFil® Precision Micro-Cannula 22G x 40mm
The SoftFil® Precision 22G x 40mm Micro-Cannula is a high-precision, single-use sterile cannula designed for controlled filler delivery and mesotherapy procedures.
Developed by Soft Medical Aesthetics (Paris, France), this model offers exceptional flexibility, safety, and accuracy, making it ideal for mid-depth injections and aesthetic rejuvenation treatments.
Product Description
The SoftFil® Precision 22G x 40mm combines an ultra-smooth stainless-steel finish with a rounded atraumatic tip, ensuring patient comfort while reducing the risk of bruising and swelling.
This cannula provides excellent maneuverability and precision, making it particularly suitable for facial contouring, nasolabial folds, and tear trough rejuvenation.
Its 40mm length allows for controlled injection of fillers and regenerative materials with natural, even diffusion.
Technology & Composition
Material: Surgical-grade stainless steel cannula with a highly polished micro-finish for smooth insertion
Tip Design: Rounded atraumatic tip with side port for homogeneous filler diffusion
Markings: Laser-engraved centimeter indicators for precise depth control
Wall Type: Thin-wall engineering optimizing flow for low to medium viscosity products
Introduction Needle: Includes a sharp 22G introducer needle for accurate and safe pre-puncture
Sterilization: Sterilized using ethylene oxide (EO) according to European medical device standards
Packaging
Box of 20 sterile units
Each cannula is individually packaged in a medical-grade blister pouch
Includes a matching 22G introducer needle per unit
Indications & Uses
Medium-depth dermal and subcutaneous injections
Facial contouring and rejuvenation
PRP and mesotherapy applications
Nasolabial folds, marionette lines, and tear trough correction
Collagen induction and skin bio-remodeling treatments
Clinical Benefits
Reduced patient trauma through atraumatic rounded-tip design
Precise control with laser depth markings
Enhanced flow rate for smooth, efficient injection
Uniform diffusion of filler or bioregenerative materials
Reliable and safe for aesthetic professionals
Manufacturer & Regulatory Information
Manufacturer: Soft Medical Aesthetics
Manufacturing Location: Paris, France
Compliance:
• CE Marked (Class IIa Medical Device) under EU Regulation (MDR) 2017/745
• ISO 13485 Certified Manufacturer
• Distributed under compliance with AEMPS (Agencia Española de Medicamentos y Productos Sanitarios) in Spain and the European Union
For Professional Use Only:
As trusted distributors and providers of aesthetic medicine, Aesthisave® emphasizes that our products are strictly for professional use.
We insist that these treatments be administered only by trained and medically qualified personnel, ensuring the highest standards of safety, precision, and patient care.