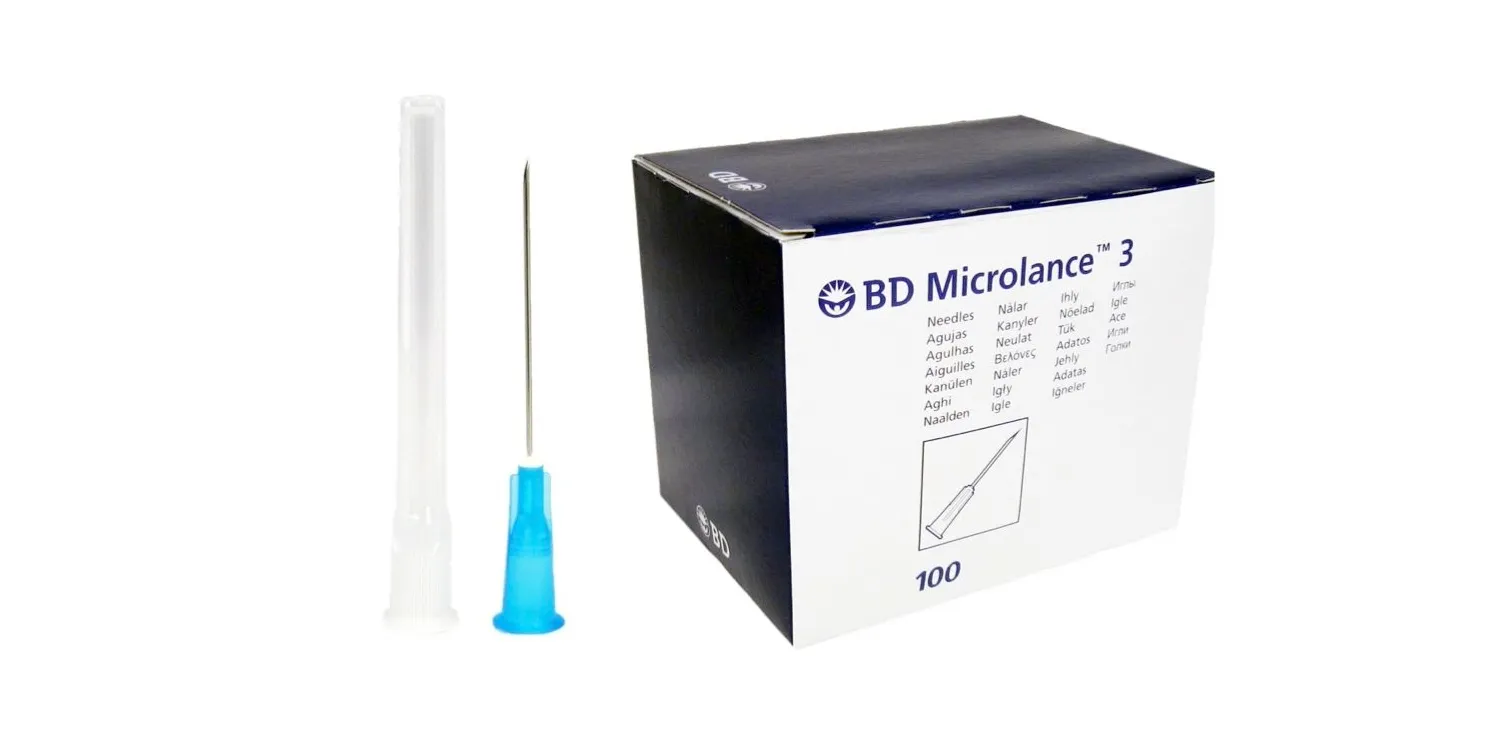
BD MICROLANCE™ 3 HYPODERMIC NEEDLE 23G x 30MM
The BD Microlance™ 3 Hypodermic Needle 23G x 30mm is a precision-engineered medical needle designed for optimal performance in a variety of injection and aspiration procedures.
Crafted from surgical-grade stainless steel with a triple-bevel ultra-sharp tip and silicone coating, it provides smooth penetration, enhanced flow, and superior comfort — ideal for both medical and aesthetic applications.
Product Description
Type: Hypodermic needle for single use
Gauge / Color Code: 23G – Blue (EN ISO 6009)
Length: 30 mm (1 ¼”)
Tip Design: Ultra-sharp triple-bevel lancet point for precise puncture and reduced resistance
Material: Stainless steel cannula with color-coded polypropylene hub
Sterility: Individually blister-packed, sterilized with Ethylene Oxide (EO)
Compatibility: Fits Luer Slip and Luer Lock syringes
Latex-Free / PVC-Free: Safe for all patient types and eco-friendly
Technology & Composition
Cannula: Surgical-grade AISI 304 stainless steel ensuring strength, flexibility, and corrosion resistance
Wall Design: Thin-walled cannula with an enlarged lumen to enhance fluid flow for both injection and aspiration
Tip Geometry: Triple-bevel precision sharpening ensures reduced penetration force and minimal tissue trauma
Surface Coating: Medical-grade silicone for smooth insertion and comfort
Hub Design: Transparent, color-coded polypropylene hub for easy gauge recognition
Manufacturing Precision: Laser-controlled beveling and automatic inspection guarantee consistency and safety
Compliance Standards: Conforms to ISO 7864 and EN ISO 6009
Packaging
Quantity: Box of 100 individually blister-packed sterile needles
Sterilization: Ethylene Oxide (EO)
Shelf Life: 5 years under recommended storage conditions
Traceability: Each unit includes batch number and expiry date for quality assurance
Indications & Uses
Designed for intradermal, subcutaneous, intramuscular, and intravenous injections, as well as aspiration procedures
Suitable for medical, dental, and aesthetic treatments such as botulinum toxin injections, mesotherapy, and local anesthetic delivery
Compatible with all Luer Slip and Luer Lock syringes
Clinical Benefits
Smooth Performance: Thin-wall design enhances fluid delivery and withdrawal rates
Comfortable Treatment: Triple-bevel tip and silicone lubrication reduce pain and tissue trauma
Durable & Reliable: Stainless steel cannula maintains sharpness and rigidity for consistent results
Safe & Hygienic: Single-use, sterile, and latex-free
Professional Versatility: Trusted by healthcare and aesthetic professionals worldwide
Manufacturer & Regulatory Information
Manufacturer: Becton, Dickinson and Company (BD Medical)
Manufacturing Location: Fraga, Spain / Le Pont-de-Claix, France (EU facilities)
Compliance:
• CE Marked (Class IIa Medical Device) under EU Regulation (MDR) 2017/745
• ISO 13485 & ISO 9001 Certified Manufacturer
• Conforms to ISO 7864 and EN ISO 6009
• Distributed under compliance with AEMPS (Agencia Española de Medicamentos y Productos Sanitarios) in Spain and the European Union
For Professional Use Only:
As trusted distributors and providers of aesthetic medicine, Aesthisave® emphasizes that our products are strictly for professional use.
We insist that these treatments be administered only by trained and medically qualified personnel, ensuring the highest standards of safety, precision, and patient care.