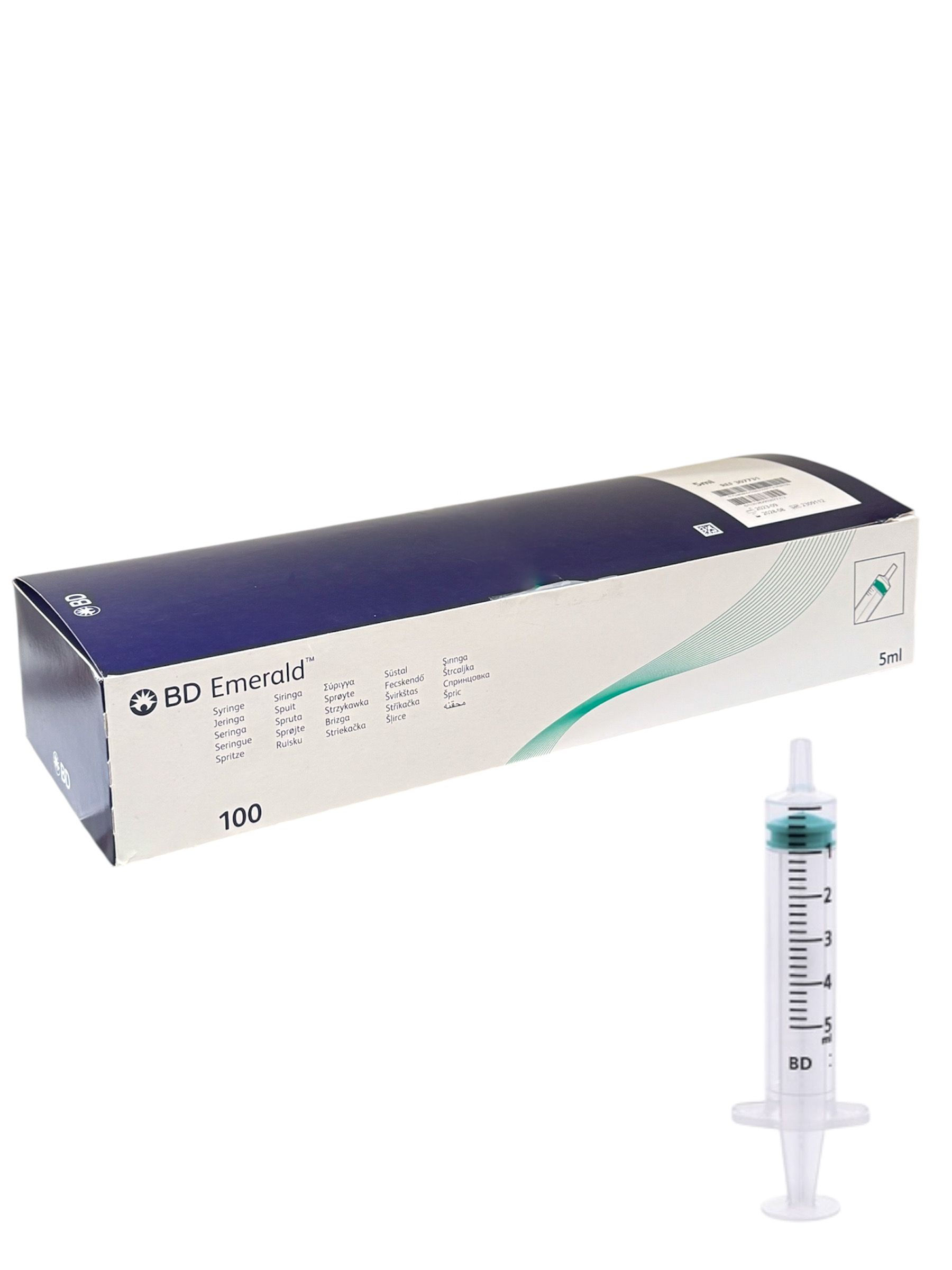
BD EMERALD™ 5 ml 3-Part Luer Slip Syringes
The BD Emerald™ 5 ml 3-Part Luer Slip Syringe is a precision-engineered, single-use syringe designed for injections and infusions across medical and aesthetic practices. Manufactured by Becton, Dickinson and Company (BD), it combines accuracy, patient safety, and sustainability, reflecting BD’s ongoing commitment to eco-efficient healthcare solutions.
Product Overview
Engineered for both safety and environmental responsibility, the BD Emerald™ syringe range is manufactured using up to 30 % less material than standard syringes, significantly reducing medical waste. The green stopper improves contrast and visibility against the clear barrel, ensuring precise dosage and controlled administration.
Technical Specifications & Composition
Volume: 5 ml
Type: Luer Slip (needle not included)
Design: 3-part syringe – barrel, plunger, and latex-free stopper
Graduation: 0.2 ml increments for accurate dosing
Stopper Colour: Green for enhanced visibility
Material: Latex-free, medical-grade polymers
Sterility: Individually sterile-packed, single use
Packaging: Box of 100 individually sterile syringes
Compliance: CE-marked, ISO 7886-1, ISO 549 compliant
Indications & Applications
Injections: Suitable for administration of aesthetic injectables and medical solutions
Infusions: For infusion preparation and dilution procedures
Aspiration: For aspirating or withdrawing medical fluids
Clinical & Functional Benefits
Eco-Responsible Design: Manufactured using 30 % less material for reduced environmental impact.
Enhanced Precision: Green stopper and clear scale markings ensure accurate volume control.
Latex-Free Safety: Minimizes the risk of allergic reactions.
Proven Quality: Each syringe meets over forty BD-specific performance and safety criteria.
Usage Instructions
Preparation: Attach a compatible sterile Luer Slip needle (sold separately).
Filling: Draw the desired amount of medication, checking the clear, easy-to-read scale.
Administration: Inject or aspirate as needed, maintaining full control during the procedure.
Disposal: Discard after single use following local sharps and medical waste regulations.
Manufacturer & Regulatory Information
Manufacturer: Becton, Dickinson and Company (BD Medical)
Manufacturing Location: Fraga, Spain / Le Pont-de-Claix, France (EU manufacturing sites)
Compliance:
• CE Marked Class IIa Medical Device under EU Regulation (MDR) 2017/745
• ISO 13485 & ISO 9001 Certified Manufacturer – compliant with ISO 7886-1 standard for sterile hypodermic syringes
• Distributed under compliance with AEMPS (Agencia Española de Medicamentos y Productos Sanitarios) in Spain and the European Union
For Professional Use Only:
As trusted distributors and providers of aesthetic medicine, Aesthisave® emphasizes that our products are strictly for professional use.
We insist that these treatments be administered only by trained and medically qualified personnel, ensuring the highest standards of safety, precision, and patient care.